- Teacher: أميرة عبدالمجيد أبوطالب اللبودي .
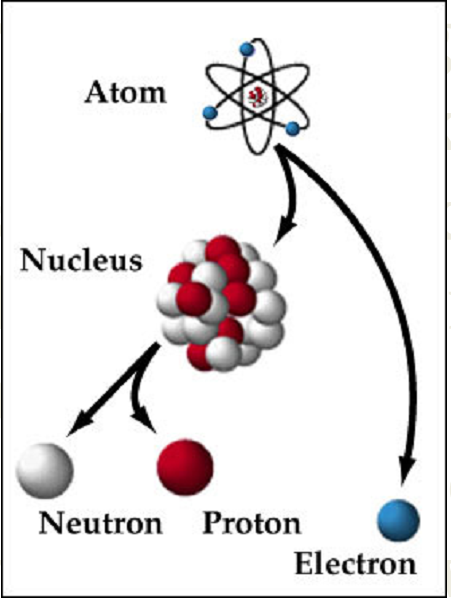
Atomic physics may loosely be defined as the scientific study of the structure of the atom, its energy states, and its interactions with other particles and fields. Learning Atomic Physics is important not only for understanding the physics of the atom but also the technological applications thereof. For example, the fact that each element has its own characteristic “fingerprint” spectrum has contributed significantly to advances in material science and also in cosmology. The aim of this course is to guide the learner through a chronological development of Atomic Physics. The learner begins by studying the development of atomic models from Dalton, Thompson, Rutherford and finally Bohr. After atomic models the learner is guided through a phenomenon that led to the discovery of the electron and its negative charge. Gas discharge experiments also laid the ground as to how atoms could be excited.
-------------------------------------------------------------
----------------------------------------------------------------
-------------------------------------------------------------
----------------------------------------------------------------
- Teacher: نجلاء أحمد محمد شاهين .

